Join us for the second annual Accuray Exchange Showcase: Conference Highlights from 2025, your opportunity to explore the impactful research and innovations using the CyberKnife® and Radixact® Systems, presented at leading global conferences, including ESTRO, RSS, ISRS, AAPM and ASTRO. Beginning February 2nd, this four-day event will feature four daily sessions, each designed to give you a comprehensive overview of cutting-edge technologies, clinical applications and emerging trends shaping the future of radiation oncology.
Each webinar is pending approval by CAMPEP (MPCEC) and ASRT (Category A credit), with credits determined by the webinar duration.
- Mon, Feb 2nd
- Tues, Feb 3rd
- Wed, Feb 4th
- Thurs, Feb 5th
Day 1: CNS Indications

University of Bologna, IRCCS Institute of Neurological Sciences of Bologna, Italy
4:00 PM
Presenter: Alfredo Conti, M.D., Ph.D., FEBNS (University of Bologna, IRCCS Institute of Neurological Sciences of Bologna, Italy)
4:05 PM
Presenter: Pantaleo Romanelli, M.D. (Renaissance Institute of Stereotactic Radiosurgery and Precision Oncology, Winter Park, Florida , USA)
4:15 PM
4:25 PM
Neuroanatomical Synergy in Cyberknife Stereotactic Ablative Neuromodulation: Revisiting Contouring Paradigms with Dopamine PET-CT and Fgatir MRI for Ventral Intermediate Nucleus Targeting in Parkinson’s Disease for Improved Outcomes
4:35 PM
Hypofractionated radiosurgery for functioning and non-functioning pituitary adenomas after surgery: Our working experience with Cyberknife unit.
4:45 PM
Single center clinical experience with Cyberknife radiosurgery for skull base meningiomas
4:55 PM
Long-Term Outcomes of Vestibular Schwannoma Treated with Stereotactic Radiosurgery: A Retrospective Study from a Single Institution
Presenter: David J. Park, MD, Ph.D., FCNS (Stanford University School of Medicine · Palo alto, USA)
5:05 PM
Multi-sequential stereotactic radiosurgery (SRS) for brain metastases: 10-year experience from the CHUV (Lausanne, Switzerland) brain metastasis clinic.
Presenter: Luis Schiappacasse, M.D. (CHUV, Lausanne, Switzerland)
5:15 PM
Treatment of glomus tumor with CyberKnife.
Presenter: Zeno Perini (San Bortolo Hospital, Vicenza, Italy)
Day 2: Breast and Gynecological Cancers

U.T. Southwestern Medical Center, Dallas, TX, USA
4:00 PM
Welcome
Presenter: Asal Rahimi, M.D., MS, (U.T. Southwestern Medical Center, Dallas, TX, USA)
4:05 PM
Stereotactic Radiotherapy Boost in Locally Advanced Cervical Carcinoma Patients (STARBACS): Up-to-Date Results of a Phase II, Single Arm, Monoinstitutional Study
Presenter: Giacomo Ferrantelli, M.D. (University of Messina, Messina, Italy)
4:15 PM
Evaluation of New SGRT-Based Approach to DIBH for Left-Sided Breast Cancers on a Helical Delivery Platform
Presenter: Sanjay Hunugundmath, M.D. (Sahyadri Superspecialty Hospital, Pune, India)
4:25 PM
Automated Deep Inspiration Breath-Hold (DIBH) in Breast Radiotherapy: A Comprehensive Assessment of the VitalHold System on the Radixact Platform
Presenter: Paul RETIF, Ph.D. (CHR Metz-Thionville, Metz, France)
4:35 PM
Long-term follow-up results of helical tomotherapy for non-metastatic breast cancer: single center experience
Presenter: Abdelkarim Uakkas, M.D. (Institute Curie, Paris, France)
4:45 PM
Five-Year Outcomes of Robotic Stereotactic Accelerated Partial Breast Irradiation for Early-Stage Breast Cancer
Presenter: Rachelle Lanciano, M.D. (Crozer Keystone Healthcare System, Department of Radiation Oncology, Havertown, PA)
4:55 PM
Phase I Pre-Operative Single Fraction Dose Escalation Ablative Stereotactic Partial Breast Irradiation (S-PBI) Trial for Early-stage Breast Cancer
Presenter: Asal Rahimi, M.D., MS (U.T. Southwestern Medical Center, Dallas, TX, USA)
Day 3: Gastrointestinal (GI) and Genitourinary (GU) Cancers

Tata Memorial Centre, Mumbai, India
4:00 PM
Welcome
Presenter: Vedang Murthy, M.D., DNB, DipEPP (Tata Memorial Centre, Mumbai, India)
4:05 PM
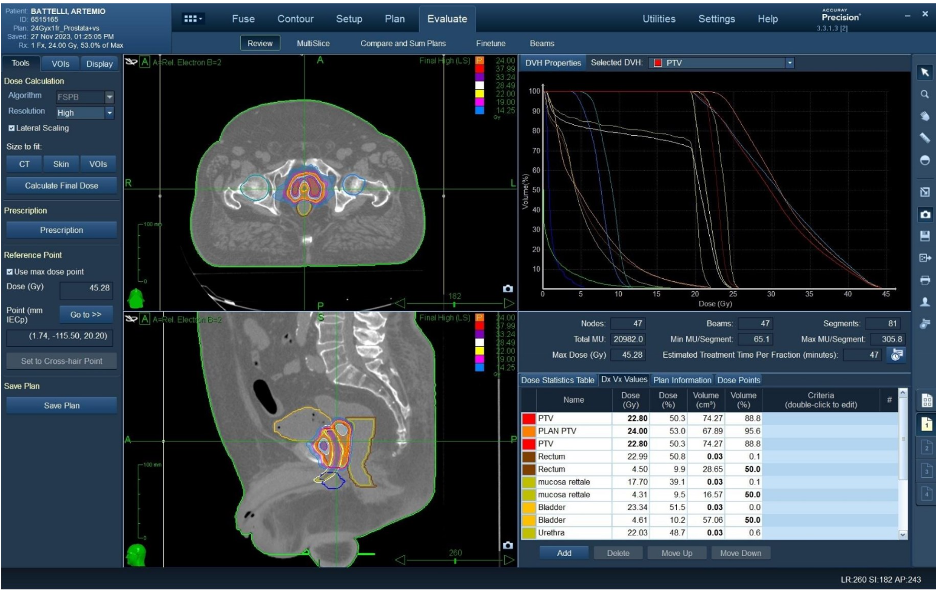
One-day urethral-sparing, HDR-like, prostate cancer robotic SBRT: preliminary results of the PRO-FAST trial
Presenter: Andrei Fodor, M.D. (IRCCS San Raffaele Scientific Institute, Milan, Italy)
4:15 PM
A Phase I/Ib, Single Arm Study of Two Fraction SBRT with SIB for the Treatment of Localized Prostate Cancer: Early Toxicity Outcomes
Presenter: Jonathan Lischalk, M.D. (Georgetown University Hospital, Washington, D.C., USA)
4:25 PM
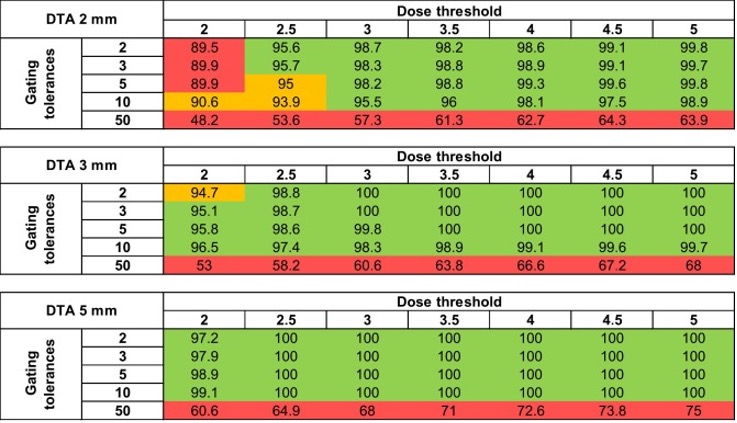
To Space or Not to Space: The EPIC question for Prostate Stereotactic Radiation (SBRT) with or without Hydrogel Rectal Spacer (RS)
Presenter: Madeline Flanagan (Dan L. Duncan Comprehensive Cancer Center, Baylor College of Medicine, Houston, TX, USA)
4:35 PM
Frameless Robotic Radiosurgery System Stereotactic Body Radiation Therapy (SBRT) Dose Escalation Prostate Cancer Trial (CK-DESPOT) for Unfavorable Intermediate and High-Risk Prostate Cancer
Presenter: Rachelle Lanciano, M.D. (Crozer Keystone Healthcare System, Department of Radiation Oncology, Havertown, PA, USA)
4:45 PM
Two-Year Toxicity Outcomes from Pace-C: Stereotactic Body Radiotherapy (SBRT) Versus Moderate Hypofractionated Radiotherapy (MHRT)
Presenter: Ragu Ratnakumaran, M.D. (The Royal Marsden NHS Foundation Trust, London, United Kingdom)
4:55 PM
Bladder Adjuvant Radiotherapy (BART): Clinical Outcomes from a Phase III Multicenter Randomized Controlled Trial
Presenter: Vedang Murthy, M.D., DNB, DipEPP (Tata Memorial Centre, Mumbai, India)
Day 4: Extracranial Metastatic Disease

European Institute of Oncology, Milan, Italy
4:00 PM
Welcome
Presenter: Barbara Alicja Jereczek-Fossa, M.D., Ph.D. (European Institute of Oncology, Milan, Italy)
4:05 PM
A review of 130 Cyberknife spinal metastasis SABR treatments at one centre to assess risk of spinal cord toxicity
Presenter: Timothy Jackson (Queen Elizabeth Hospital, Birmingham, United Kingdom)
4:15 PM
Efficacy and Safety of Donut-Shaped Circumferential Spine CyberKnife Stereotactic Body Radiotherapy for Metastatic Spine Disease
Presenter: David J. Park, MD, PhD, FCNS (Stanford University School of Medicine, Palo Alto, USA)
4:25 PM
Stereotactic Body Radiotherapy (SBRT) Boost Following Urgent 3D Conformal Radiotherapy in the Treatment of Metastatic Epidural Spinal Cord Compression (MESCC): A Phase I Feasibility Trial
Presenter: Elysia Donovan, M.D. (McMaster University – Juravinski Cancer Centre, Hamilton, ON, Canada)
4:35 PM
Use of Stereotactic Body Radiotherapy (SBRT) for Spine Metastases with Robotic Radiosurgery Unit Cyberknife in Patients with Oligometastatic Disease
Presenter: Eva Fernandez Lizarbe, M.D. (H. U. Ramón y Cajal, Madrid, Spain)
4:45 PM
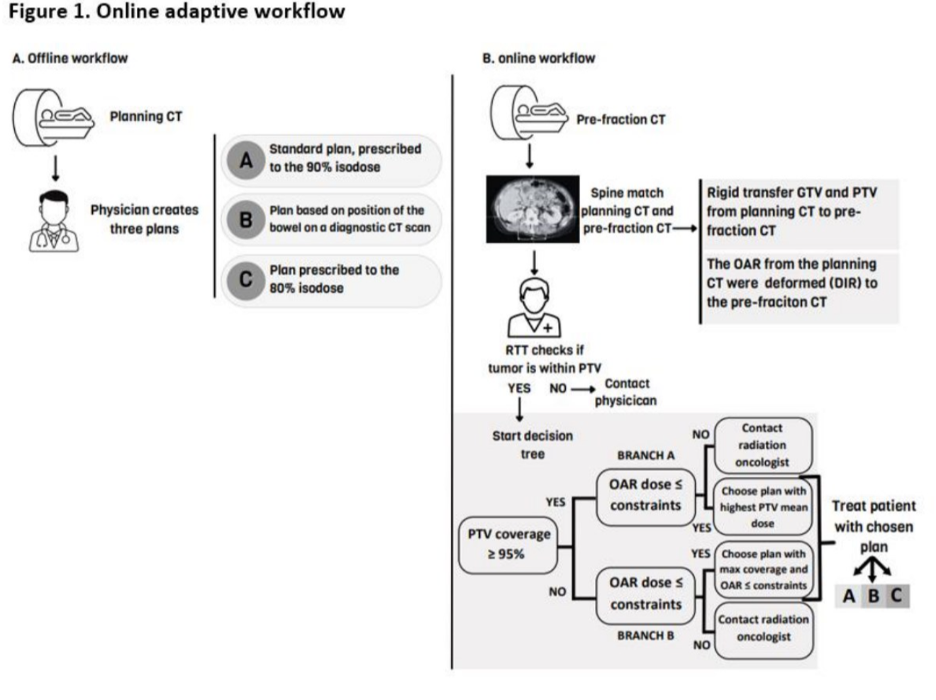
Increased iso-toxic dose to the target in oligometastatic abdominal lymph nodes using CT-guided online adaptive SBRT
Presenter: Lucy A. van Werkhoven, M.D. (Erasmus MC Cancer Institute, University Medical Center, Rotterdam, Netherlands)
4:55 PM
Stereotactic Body Radiotherapy for the Treatment of Oligometastases Located in the Peritoneum or in the Abdominal Wall: Preliminary Results from a Mono-Institutional Analysis
Presenter: Francesco Cuccia, M.D. (ARNAS Civico Hospital, Palermo, Italy)
5:05 PM
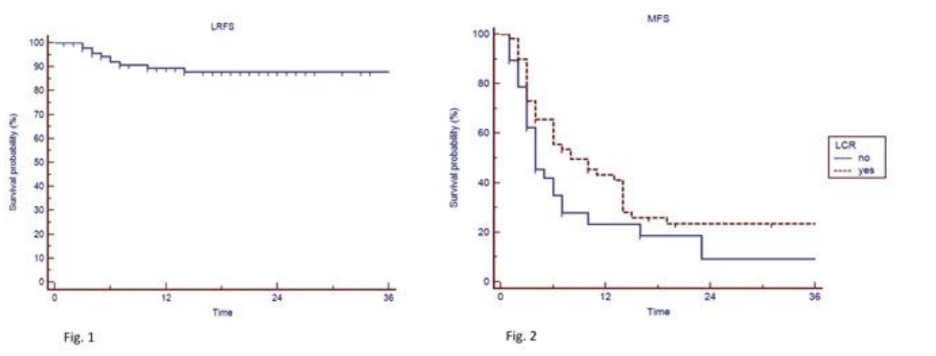
Higher disease-free survival observed for complete response after SBRT in oligometastatic gynecologic tumors
Presenter: Gaia Parma, M.D. (IRCCS San Raffaele, Milan, Italy)
5:15 PM
The Changing Dogma in Oligometastatic Renal Cell Carcinoma – SBRT
Presenter: Papaiah Susheela Sridhar, M.D. (Apollo Cancer Centre, Bengaluru, India)