Missed the opportunity to watch the AEx Showcase live? Catch-up at your convenience:
Day 1: CNS Indications
Day 2: CNS Indications
Day 3: Breast and Gynecological Cancers
Day 4: Thorax and Rare Indications
Day 5: Genitourinary and Gastrointestinal Cancers
Watch the highlights now:
- Mon, Feb 3rd
- Tues, Feb 4th
- Wed, Feb 5th
- Thurs, Feb 6th
- Fri, Feb 7th
Day 1: CNS Indications
Safety and efficacy of CyberKnife radiosurgery plus anlotinib hydrochloride in patients with recurrent glioblastoma: a prospective phase II single-arm study (HSCK-002)
Helical TomoTherapy Compared to Intensity-Modulated Radiation Therapy in Hippocampal Avoidance Prophylactic Cranial Irradiation in Patients with Limited-Stage Small-Cell Lung Cancer
Presenter: Hui Zhu (Shandong Cancer Hospital and Institute, China)
Stereotactic Radiosurgery and Radiotherapy for Brainstem Metastases: An International Multicenter Analysis
Tumor Control Probability and Time-Dose Response Modeling for Stereotactic Radiosurgery of Uveal Melanoma
Neurocognition and Quality of Life for Hypofractionated Stereotactic Radiotherapy (HFSRT) of the Resection Cavity vs. Whole-Brain Radiotherapy (WBRT) Following Brain Metastasis Resection
Presenter: Rami El Shafie (University Medical Center Gottingen, Germany)
Hypofractionated radiosurgery for residual/ recurrent non secreting pituitary adenomas an exploratory study: preliminary results
Day 2: CNS Indications
Accuray Exchange President welcome and chair introduction
Presenter: Sean Collins (Tampa General Hospital, USA)
Presenter: Pantaleo Romanelli (Renaissance Institute of Precision Oncology & Radiosurgery, USA)
Stereotactic diffusion tensor imaging tractography for brain AVM located in the in deep seated eloquent areas during radiosurgery treatment planning
Presenter: Enmin Wang (Huashan, China)
A proof of concept for MR-only workflow in CyberKnife intracranial radiosurgery
Presenter: Evaggelos Pantelis (National and Kapodistrian University of Athens, Greece)
Stereotactic radiosurgery in choroidal hemangioma with CyberKnife
Presenter: Kaan Oysul (Ankara, Turkey)
Simultaneous Multiple Brain Metastases SRS/SRT: An Evaluation of Dose Coverage Uncertainty Induced By Intra-Fraction Patient Motion during Beam Delivery
Presenter: Xiaoming Chen (Fox Chase, USA)
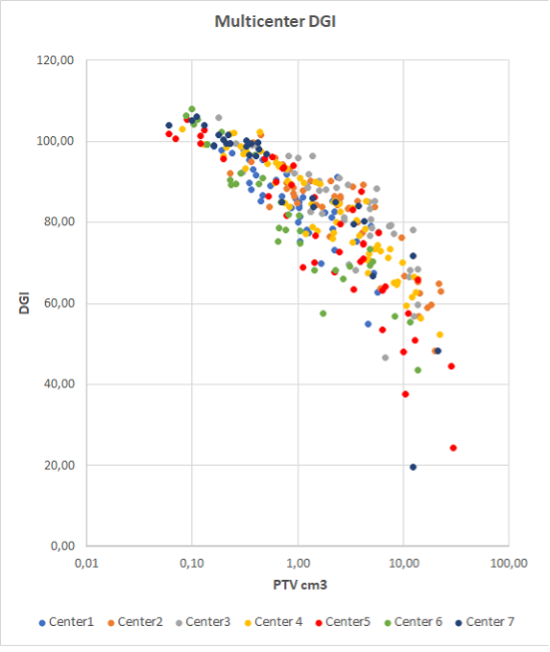
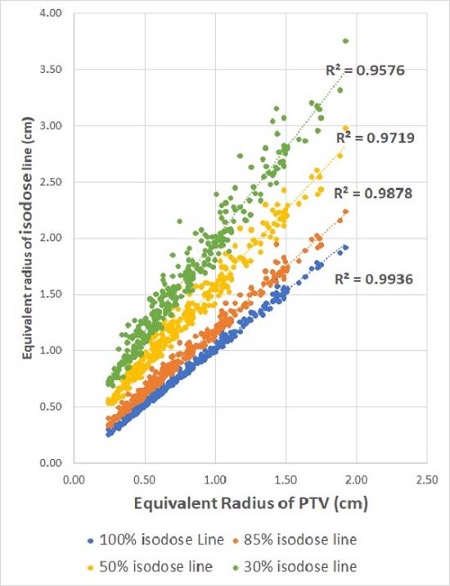
Multicenter approach to guide plan optimization of robotic intracranial SRS/SRT
Presenter: Sara Broggi (San Raffaele, Italy)
Retreatment for Resistant or Recurrent Pain in Trigeminal Neuralgia Using Frameless LINAC Radiosurgery
Presenter: Pantaleo Romanelli (Milan, Italy)
Clinical outcome and dosimetric feasibility of hippocampal sparing whole brain radiotherapy
Presenter: Bhuvana J. (Ahmedabad, India)
Day 3: Breast and Gynecological Cancers
Chief Medical Officer welcome and chair introduction
Presenter: Seth Blacksburg (Accuray)
Presenter: Barbara Jereczek-Fossa (University of Milan & European Institute of Oncology, Italy)
Research on Thread Effect in HT Radiotherapy for Cervical Cancer
Presenter: Li Guang (Chongqing University Cancer Hospital, China)
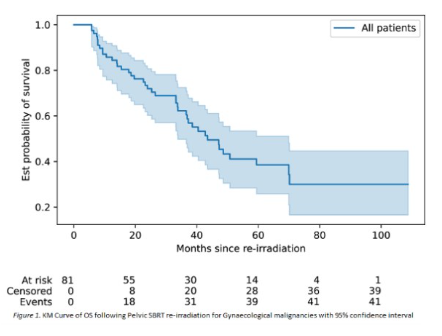
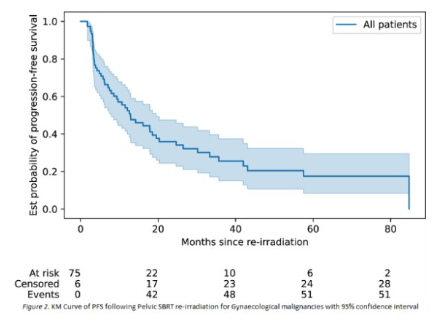
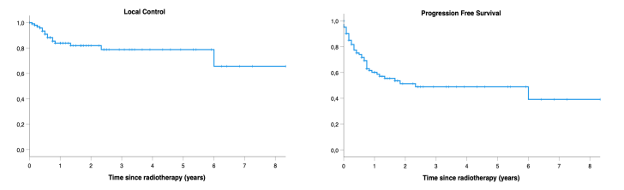
Outcomes of pelvic reirradiation with stereotactic radiotherapy for gynaecological cancer recurrence
Presenter: Susan Lalondrelle (The Royal Marsden NHS Foundation Trust, UK)
CYBERNEO trial: Update of results at 14 years of follow-up
Moderate hypofractionation with simultaneous integrated boost after conservative surgery for Bca
Presenter: Roberta Tummineri (San Raffaele, Italy)
Stereotactic Partial Breast Irradiation: 4-year Clinical Results and Cosmetic Outcomes
Presenter: Norbert Meszaros (National Institute of Oncology, Hungary)
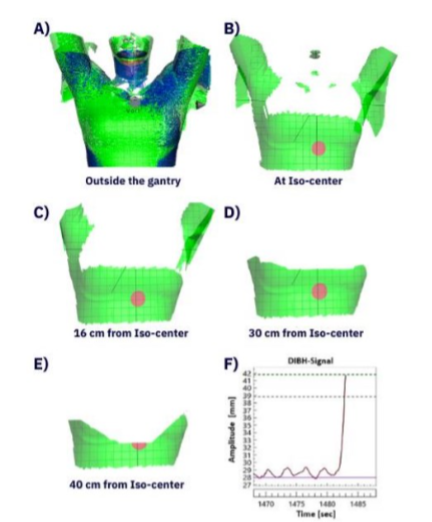
Surface guided ring gantry radiotherapy in deep inspiration breath hold for breast cancer patients
Presenter: Mustafa Kadhim (Skåne University Hospital, Sweden)
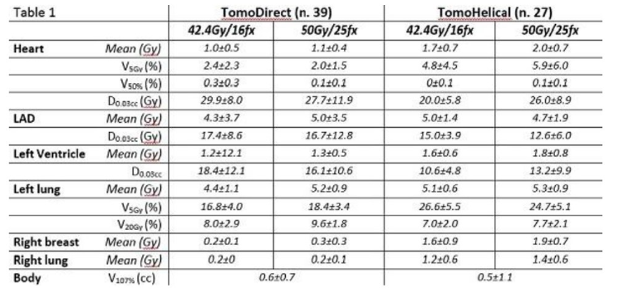
Left breast cancer dosimetry with Accuray VOLO Ultra optimizer: a comparison with DIBH
Presenter: Patrizia Urso (Gruppo Ospedaliero Moncucco, Italy)
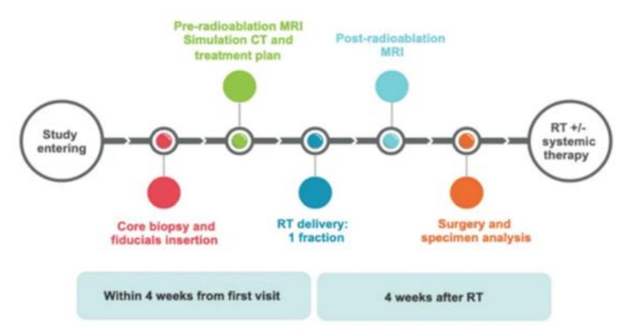
Preoperative single-fraction RT for early stage BC: preliminary results from CRYSTAL phase I/II study
Presenter: Maria Zerella (IEO, Italy)
Day 4: Thorax and Rare Indications
Accuray Exchange Executive Board welcome and chair introduction
Presenter: Jun Yang, Ph.D.., JunXin Oncology Group, Foshan, Guangdong, China
Chair welcome
Presenter: Umberto Ricardi, University of Turin, Italy
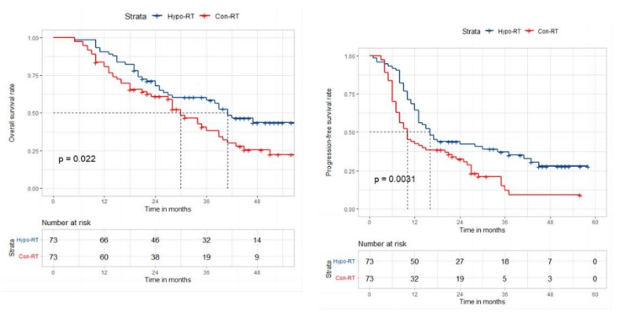
Efficacy and Toxicity of Moderately Hypofractionated Radiotherapy Via Helical TomoTherapy Versus Conventional Radiotherapy Combined with Concurrent Chemotherapy for Patients with Unresectable Stage III Non-small Cell Lung Cancer: A Multicenter, Randomized Phase III Trial
Presenter: XiaoHong Xu (Zhongshan Hospital, China)
Optimization of Treatment Plan Parameters Used in Helical TomoTherapy for Small Cell Lung Cancer Patients with Extensive Pleural Metastasis
Presenter: Longyan Duan (Shanghai, China)
Radiation-Related Late Toxicities Following Total Marrow Irradiation Transplant Conditioning Regimens
Presenter: Colton Ladbury (City of Hope, USA)
Clinical outcomes following stereotactic ablative body radiotherapy to central lung tumours
Presenter: Julie Duong (Mount Vernon Cancer Centre, UK)
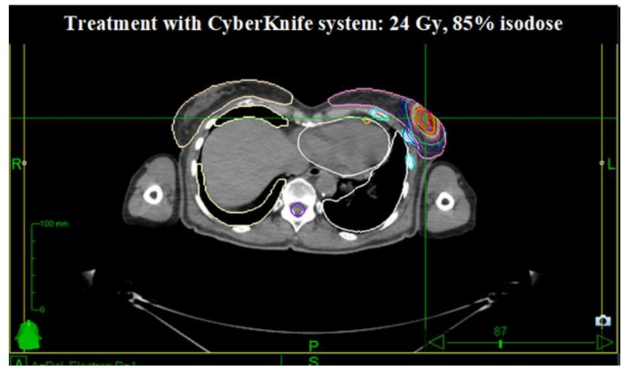
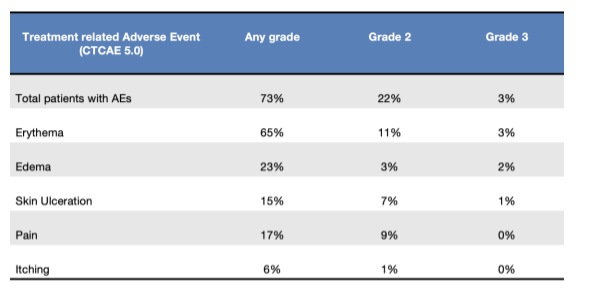
Modern radiotherapy for extended cutaneous lesions from lymphomas: results from a multicenter study
Presenter: Mario Levis (University of Torino, Italy)
Phase II Trial of TMLI 20 Gy in Combination with Cyclophosphamide and Etoposide in Patients with Poor-Risk Acute Leukemia
Presenter: Jeffrey Wong (City of Hope, USA)
Day 5: Genitourinary and Gastrointestinal Cancers
Presenter: Seth Blacksburg (Accuray)
Presenter: Alison Tree (The Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London, UK)
Presenter: Nick van As (The Royal Marsden NHS Foundation Trust, UK)
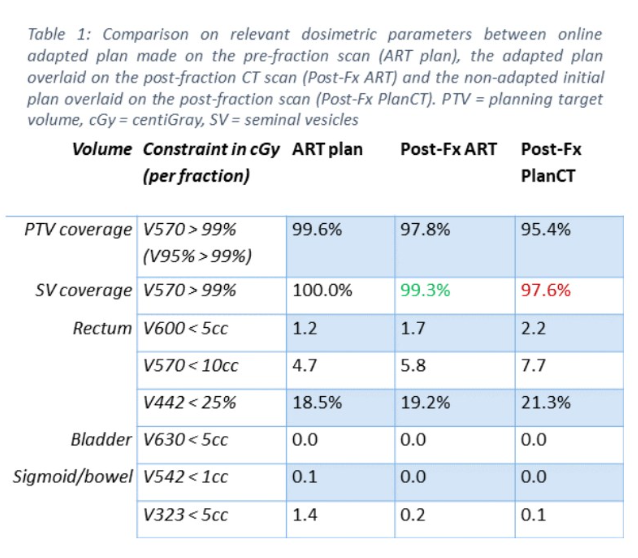
The UPRATE trial: feasibility of seminal vesicle PTV-margin reduction with online adaptive SBRT
Presenter: Victor Brand (Erasmus, Netherlands)
SBRT Focal Boost for Localised Prostate Cancer: Primary Outcomes of the SPARC Prospective Trial
Presenter: Binnaz Yasar (The Royal Marsden NHS Foundation Trust, UK)
Early PSA Kinetics in Patients Treated with Prostate SBRT with Intra-Prostatic Boost
Presenter: Nima Aghdam (Beth Israel, USA)
Outcomes of a phase II trial of high-dose online-adaptive SBRT for abdominal oligometastases
Presenter: Lucy A. van Werkhoven (Erasmus, Netherlands)
SBRT and systemic therapy for patients with Oligometastatic Renal Cell Carcinoma
Presenter: Miriam Torrisi (Ospedale San Raffaele, Italy)
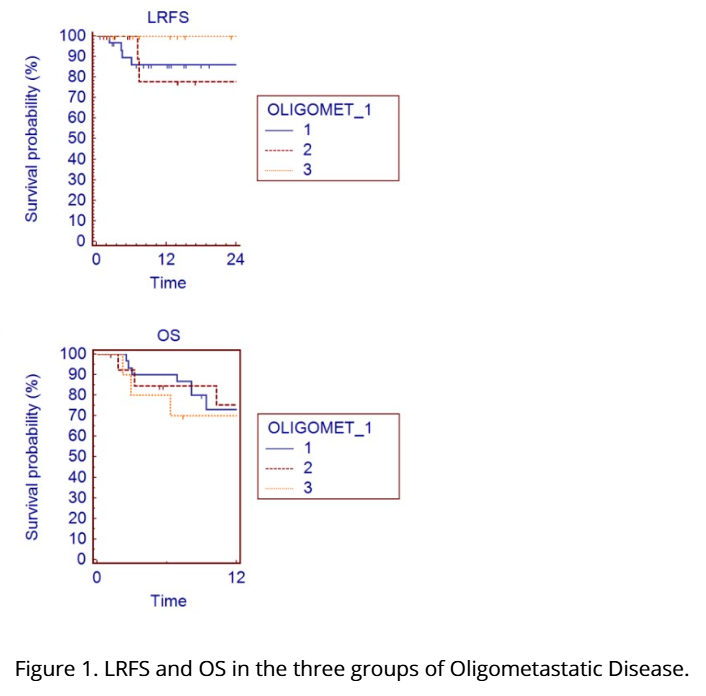
SABR for Liver De Novo, Repeat, and Induced Oligometastatic Disease
Presenter: Miriam Torrisi (Ospedale San Raffaele, Italy)
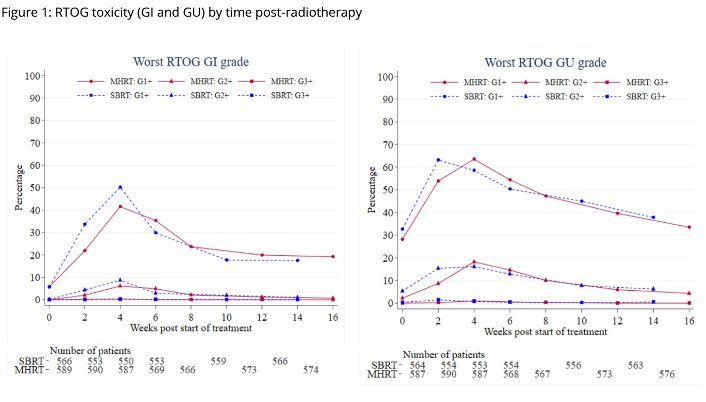
Acute toxicity from PACE-C comparing Stereotactic Body Radiotherapy (SBRT) with moderate hypofractionation (MHRT)
Presenter: Alison Tree (The Royal Marsden NHS Foundation Trust, UK)